The primary objective of this study was to develop a Filipino translation of the SMILEY and to test the validity and reliability of this translation.
Background: Systemic lupus erythematosus (SLE) is one of the most common autoimmune disorders in women of childbearing age. Simple Measure of Impact of Lupus Erythematosus in Youngsters (SMILEY) is the only health related quality of life (HRQOL) tool for pediatric SLE, which has been translated into many languages but is not yet available in Filipino.
Objective: The primary objective of this study was to develop a Filipino translation of the SMILEY and to test the validity and reliability of this translation.
Methodology: The SMILEY was translated into Filipino by a bilingual individual and back-translated by another bilingual individual blinded from the original English version. The translation was evaluated for content validity by a panel of experts and subjected to pilot testing. In the pilot, the SMILEY, together with the previously validated Pediatric Quality of Life Inventory (PEDSQL) 4.0 Generic Core Scale were administered to pediatric lupus patients and their parents on two separate occasions: a baseline and a re-test seven to fourteen days apart. Tests for convergent validity, internal consistency, and test-retest reliability were performed.
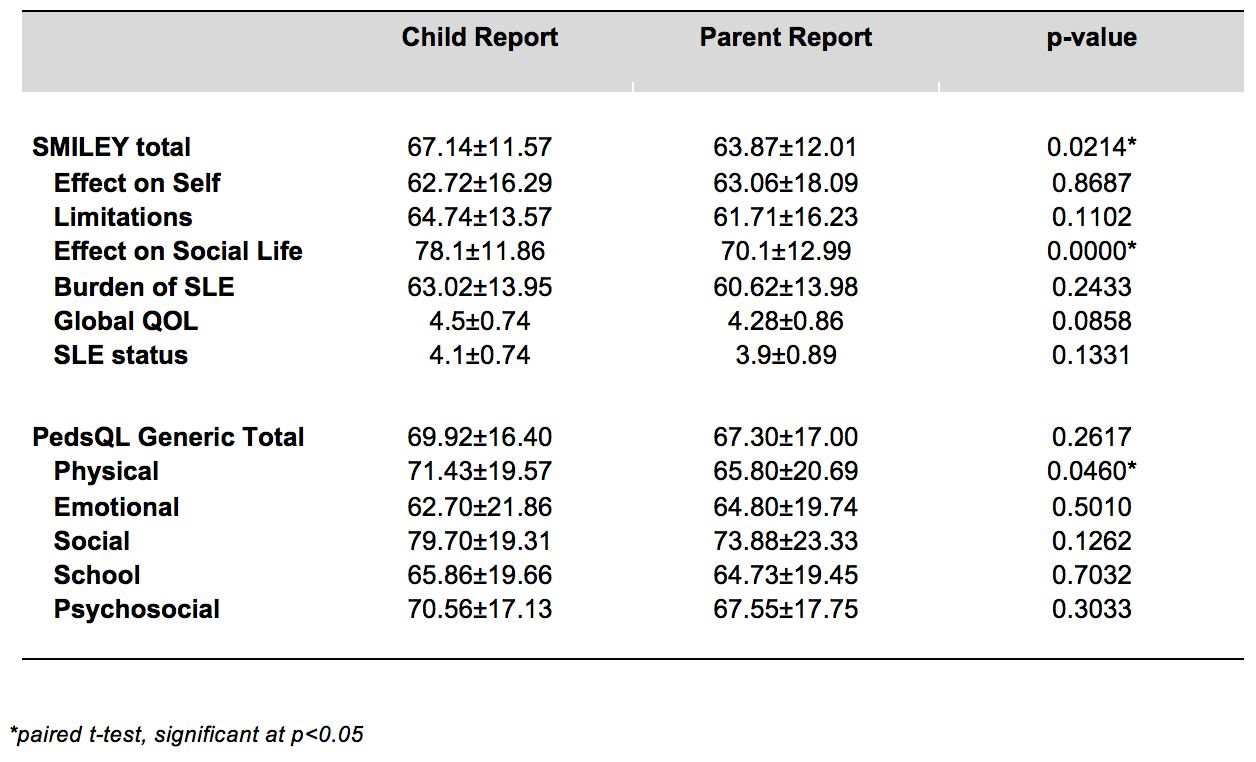
Results: A total of fifty children and their parents were recruited. The mean age was 15.38±2.62 years (range 8-18 years), mean education level was high school. The mean duration of SLE was 28 months (range 1-81 months). Subjects found the questionnaires to be relevant, easy to understand and to answer. The validity of the SMILEY was demonstrated in terms of content validity, convergent validity, internal consistency, and test-retest reliability. Age, socioeconomic status and educational attainment did not significantly impact the scores. The difference between scores reported by children and parents was significant with SMILEY Total (p=0.0214), effect on Social Life (p=0.0000), and PEDSQL Physical Function (p=0.0460), with children reporting higher scores for these domains compared to their parents.
Conclusion: SMILEY is a brief, easy to understand, valid and reliable tool for assessing specific HRQOL in pediatric SLE. It will be useful in providing better care, understanding and may offer critical information regarding the effect of SLE in the quality of life of our pediatric lupus patients. It will help physician understands the needs of their patient not only on treatment of the specific disease but as well as the impact of the treatment on their daily lives.
Jemely M. PUNZALAN, Beatrice B. CANONIGO, Maria Rosario F. CABANSAG, Dennis S. FLORES, Paul Joseph T. GALUTIRA, Christine B. BERNAL, Remedios D. CHAN
Systemic Lupus Erythematosus (SLE) is an autoimmune disease. It is characterized by the production of autoantibodies which leads to immune complex deposition, inflammation and eventually permanent organ damage. It is one of the most common autoimmune disorders in women of child bearing age, with an estimated of prevalence of 14.6 to 50.8 per 100,000 persons in the United States.1 There is a female to male ratio of about 6-10:1 with peak incidence between ages 15 and 40 all all age groups are affected.1-3 The incidence of juvenile or early onset SLE varies by location and ethnicity. Studies have estimated the incidence at 0.28 to 0.9/100,000 per year in the US.2
Children and adolescents represent 15-20% of all patients with SLE.3 Presentation, clinical symptoms, and laboratory examinations maybe similar for adults and juvenile-onset SLE but a study done by Brunner et al. showed that SLE that begins in childhood is more severe than adult onset SLE, with higher rates of organ involvement and more aggressive clinical course.4 Juvenile onset SLE may also need higher doses of corticosteroids and immunosuppressive agents for disease control more often than adults.5 Treatment results in wide range spectrum of physical, functional, psychological damage that impacts the well-being children with SLE.
Over the past decades, there has been marked improvement in prognosis of juvenile onset SLE, owing to earlier diagnosis and better approaches to treatment. As a result, children live longer and enter adult life with considerable morbidity, which may be secondary to the sequelae of disease activity, side effects of medications, and comorbid conditions.5
The assessment of SLE includes by four components: accurate diagnosis, monitoring of disease activity, recording of accumulated damage, and integration of these with the patients own perceptions of health status and quality of life. Given the complex phenotype and variable disease course of SLE, all components are equally important and are essential in improving the morbidity and quality of life in patients with SLE.1
Most health related quality of life (HRQOL) measures have evolved from the World Health Organization (WHO) definition of health: a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity (WHO 1947, 1948). Quality of life has been said to refer to the social, emotional, physical well-being of patients following treatment.7 HRQOL measurement in children is complicated by many factors such as disease chronicity, unpredictable course, heterogeneity within and between patients, use of proxy respondents and other psychosocial factors.6 Moorthy LN et al, developed a pediatric SLE specific HRQOL scale that is brief, easy to understand, valid and reliable.9 A valid, reliable and easy to understand Filipino version of SMILEY will help physician to understand the needs of their patient not only regarding treatment of their disease but also regarding the impact of the treatment to their daily lives.
This study aims to create and validate a Filipino translated questionnaire of Simple Measure of the Impact of Lupus Erythematosus in Youngsters (SMILEY).
This is a validation study which consists of three phases: phase I translation of the questionnaire into the Filipino language, phase II content validity by panel of experts and pilot testing, and phase III validation and reliability testing proper.
The institutional review board and pediatrics ethics subcommittee of the hospital approvals were obtained for the study. Written and verbal consent were obtained for both parent and child.
Figure 1: Algorithm of the linguistic translation process
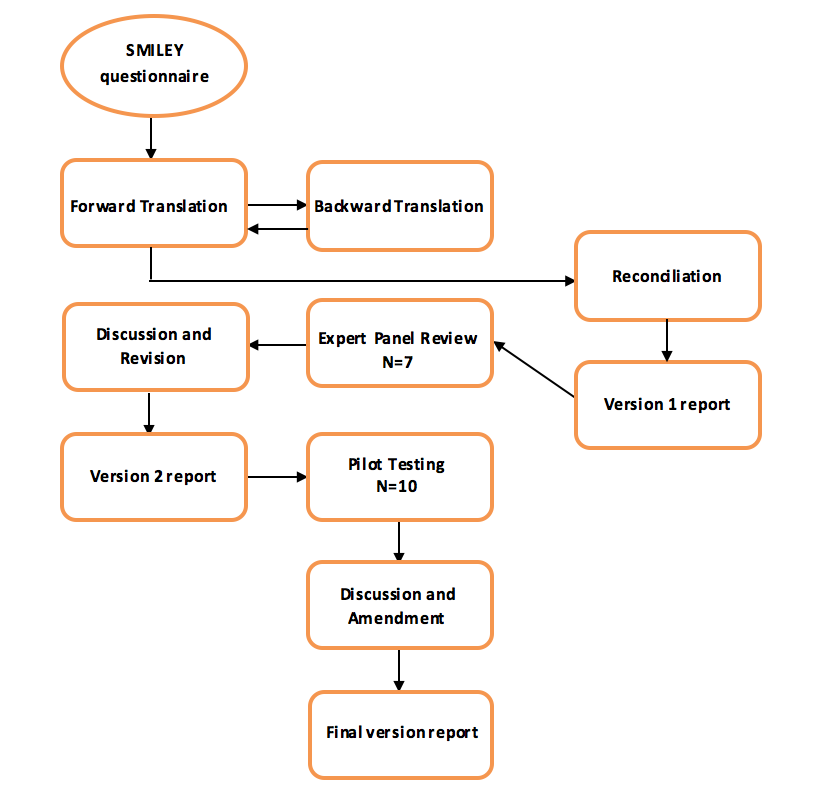
A convenience sample of 50 children aged 8-18 years old diagnosed with systemic lupus erythematosus was identified during routine clinic visits or during in-patient admissions. The participants were divided into two age groups, group I composed of subjects aged 8 to12 and group II are subjects aged 13 to 18 years. Children and parents completed appropriate questionnaires in a quiet room. Children completed their questionnaires independently of their parents.
Demographic data such as age, sex, parent and child educational attainment and socioeconomic status were recorded. Clinical data such as duration of SLE and current medication were also collected.
Questionnaires:
- Simple Measure on the Impact of Lupus Erythematosus in Youngsters Questionnaire (SMILEY)
The Simple Measure on the Impact of Lupus Erythematosus in Youngsters (SMILEY) was developed by LN Moorthy et al. which conceptualizes HRQOL as the childrens and parents perception of the impact of SLE on different aspects of childrens lives.9 It is the only disease specific HRQOL for juvenile onset SLE at present. Dr. Moorthy has granted the permission for legal use, cultural translation and validation of the SMILEY into the Filipino language. - The Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales
The Pediatric Quality of Life Inventory (PedsQL) 4.0 generic Core Scales was adopted to evaluate the generic measures of quality of life (QoL) and for the convergent psychometric validation. This is used as a gold standard for this study. Dr. James W. Varni, of the Mapi Research Institute in Lyon, France, the copyright holder has granted permission for the legal use of the questionnaire in this study.
The questionnaires were administered on two occasions. Children and parents completed the corresponding versions of SMILEY and PedsQL Generic Scale at baseline and during a subsequent follow up visit, 7-14 days later.
Data analysis was done using the Stat SE version 12 software. Descriptive statistics included mean and standard deviations for qualitative variables and frequency and percent distributions for qualitative variables.
Agreement between child and parent reports on SMILEY and PEDSQL questionnaires were determined using dependent t-test, Cronbachs alpha, Pearsons and intra-class correlations. Comparison of SMILEY and PEDSQL responses across age groups, social economic status, and educational status were compared using the independent t-test with statistical significance set at P<0.05.
All the reviewers (expert panels, parents and patients) of the questionnaire found the SMILEY to be valid, relevant, and easy to understand. The respondents thought the face form makes it easier to answer and comprehend.
The results of the evaluation by the expert panel and the pilot testing for the parent questionnaire showed mean and median scores as satisfactory (4.0) to very satisfactory (5.0) translation. The results of the evaluation by the expert panel and the pilot testing for the child questionnaire also showed mean and median scores as satisfactory (4.0) to very satisfactory (5.0) translation. The modified translation was administered to 10 random lupus pediatric patients and their parents. The composite scores reflect that the translation was easy to very easy to understand and answer. The ICC value (0.982, 0.988) indicates that there is an agreement among the raters of the parent and child group respectively. Discussion and amendment was done for the final version of the questionnaire.
Fifty child and parent subjects were included in the study proper. Forty-five (90%) were female with a mean age of 15.38±2.62 years (range 8-18 years), and the mean education was at high school level. Majority of the subjects were Catholics (86%) and belonged to low income families (66%). The mean duration of SLE was 28 months (range 1-81 months). All subjects were receiving or had previously received steroids (94% currently receiving and 6% had previously received). The most common immunomodulating drug used was cyclophosphamide (56%), followed by mycophenolate mofetil (14%) and azathioprine (4%). Hydroxychloroquine was used in 95% of subjects. Most of the parents who participated were mothers. Most subjects were from the National Capital Region (52%) where Tagalog language is widely spoken. Detailed data are tabulated in table 3.
The mean±SD scores of both the SMILEY and PEDSQL child and parent reports and their correlations where measured using paired t-test. There was a significant difference between the scores reported by the children and parents on the SMILEY total (p=0.214), effect on social life (p=0.0000) and PEDSQL physical functioning (p=0.0460). The children reported higher scores than the parents on all three domains.
In the SMILEY questionnaires, there was moderate correlation between the child and parent reports in the Limitation, effect on Social Life and the Burden of SLE domains (r=0.5-0.6; p<0.001), while a strong correlation was seen between the SMILEY Total and effect on Self domain (r=0.7; p<0.001). The intra-class correlation showed moderate to strong correlation between the parent and child reports (ICC 0.6-0.8; p<0.001).
Effect of SMILEY child and parent reports across age groups, socioeconomic status and educational attainment were not significantly different.
TABLE 1: Demographic characteristics
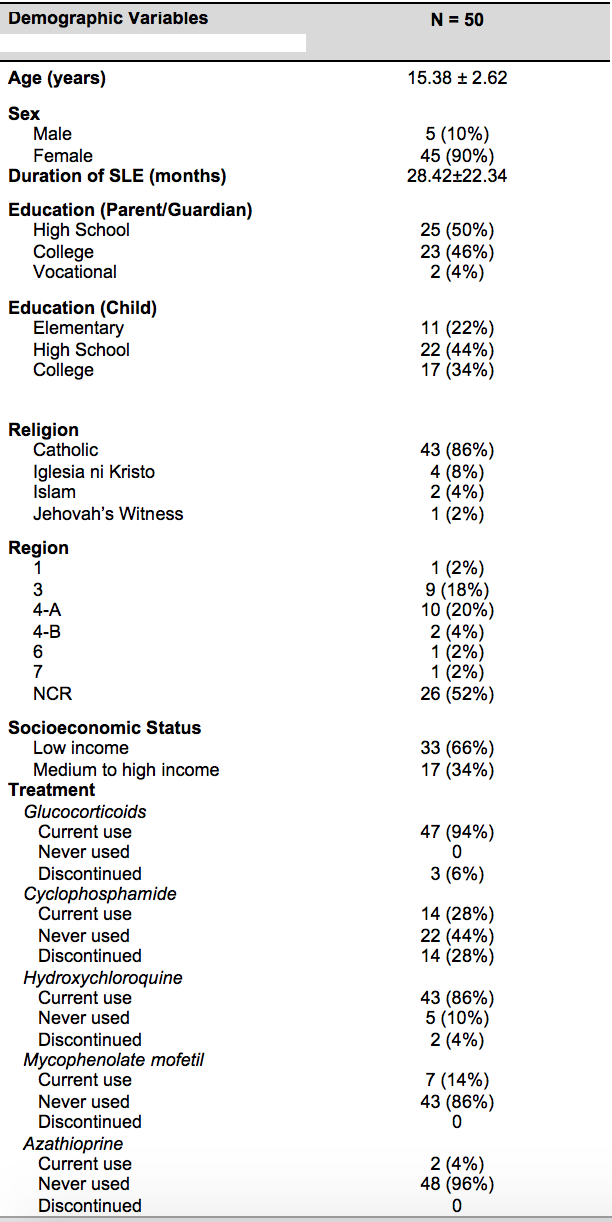
TABLE 2. Comparison between the child and parent responses to SMILEY and PedsQL questionnaires
The SMILEY child and parent report Total scores correlated significantly with reports of the PEDSQL Generic Core scale and its domains (r=0.4-0.5). However on PEDSQL parent and child reports on Physical Function (r=0.3), and parent reports on School Function (r=0.3) were only weakly correlated. There was also substantial correlation between the parent reports on Self-Perceived Global QOL (r=0.4) and the child perceived SLE Status (r=0.4).
The scale reliability coefficient for the child report of SMILEY Total and Domain scores showed good to excellent internal consistency (0.8-0.9) except for the effect on Social Life (0.6). This is comparable to the study done by Moorthy et al, were in the Cronbachs alpha for the child report SMILEY Total and Domain scores were 0.9 for Total, 0.8 for effect on Self, 0.8 for Limitation, 0.7 for Social and 0.8 for Burden of SLE.9 The parent report SMILEY Total and Domain scores were also comparable with the same study by Moorthy et al, where the scores are as follows: 0.9 for Total, 0.9 effect on Self, 0.9 for Limitation, 0.6 for Social, and 0.9 for Burden of SLE9.
Fifty child subjects and fifty parent subjects returned completed questionnaires after a mean of 11±2 days. The perceived Global QOL and SLE Status for both parent and child reports were constant at baseline and at the time of the retest confirming the test-retest reliability. The mean parent reported score at baseline was 64.07±12.15 and 63.87±12.01 on retest. The mean child reported score at baseline was 67.40±11.33 and on retest was67.14±11.57.
Determination of the quality of life of children with systemic lupus erythematosus is an important part of the holistic management of this chronic disease, and this is measurable through the use of disease specific QOL questionnaires. Our study showed that the mean scores of Filipino SMILEY total (parent 64±12, child 67±11) was comparable to the mean US SMILEY total (parent 62±16, child 65±13)9 and Brazilian SMILEY total (parent 65±15, child 69±16)19. It also showed almost similar results for other domains.This reflects that Filipino pediatric lupus patients do not have a lower quality of life despite living in a developing country with limited resources compared to patients seen and managed in developed countries like the US.
There was substantial correlation/agreement between the parent and child reports consistent with the English SMILEY. In this study, the parent reports showed lower scores in SMILEY Total score, effect in Social Life and PEDSQL Physical Functioning compared to child-reported scores. This may reflect that parents experience a greater awareness of the childs illness and worrying more about their child future. Parents may also perceive poorer QOL than their child due to their own experience, expectations, feeling of guilt, and emotions. This has been consistently noted in research comparing the reports of parents and child assessment of the childs health status. Agreement among observers has been found to be lower for subjective experiences than for observable events.20 The lack of agreement has been termed cross-informant variance and has been observed in HRQOL assessment across multiple pediatric health conditions.20 Parallel child and parent reports showed that the SMILEY was a reliable and comprehensive overall assessment of the impact of SLE in a childs quality of life. There was excellent consistency among the items and very strong reliability on re-testing, except for the parent report on the effect on Social Life.
The study population involved patients in their teenage years, more female than male, from low income families, with low disease activity and disability followed up at a tertiary hospital, which may limit generalizability of the study. Similar to other studies, the risk of children and parents conferring with each other while completing the questionnaires was unavoidable.
This study showed that the Filipino version of SMILEY was a valid and a reliable health specific quality of life measure, which was easy to understand, administer and answer for both children and parents. It was suitable across age groups, socioeconomic status and educational attainment and was comparable to the original English version of SMILEY9.
-
- Lam, G.K.W., Petri, M. Assessment of Systemic Lupus Erythematosus. Clinical Experimental Rheumatology. 2005;23:S120-132.
- Gulay, D., Dans, L. Clinical Presentations and Outcomes of Filipino Juvenile Systemic Lupus Erythematosus. Pediatric Rheumatology. 2011;9:1-7.
- Suarez, RG, Ruperto, N, et al. A Proposal Version of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index Based on th Analysis of 1,015 Patients with Juvenile Onset Systemic Lupus Erythematosus. Arthritis and Rheumatism. 2006;54(9):2989-2996.
- Brunner, HI, Feldman, BM, et al. Sensitivity of the Systemic Lupus Erythematosus Disease Activity Index, British Isles Lupus Assessment Group Index, And Systemic Lupus Activity Measure in the Evaluation of Clinical Change in Childhood-onset Systemic Lupus Erythematosus. Arthritis and Rheumatism. 1999;42:1354-60.
- Ravelli, A, Ruperto, N. Outcome in Juvenile Onset Systemic Lupus Erythematosus. Current Opinion in Rheumatology. 2005;17:568-73.
- Moorthy, LN, Peterson, MGE, et al. Relationship between Health Related Quality of Life and SLE Activity and Damage in Children over Time. Lupus. 2009;18:622-629.
- Greer S. The psychological dimension in cancer treatment. Social Science Medicine. 1984;18:345349.
- Bowling, A. Health-Related Quality of Life; Conceptual Meaning, Use and Measurement. Measuring Disease. 2nd edition; 1-22.
- Moorthy, LN, Peterson, MGE, et al. Multicenter Validation of a New Quality of Life Measure in Pediatric Lupus. Arthritis and Rheumatism. 2007;57 (7):1165-1173.
- Moorthy, LN, Peterson, et al. Quality of Life in Children with Systemic Lupus Erythematosus. Current Rheumatology Rep. 2005; 7:447-452.
- Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients.The Committee on Prognosis Studies in SLE. Arthritis & Rheumatism 1992; 35: 630-40.
- Gerber L.H. Measures of Function and Health-Related Quality of Life In Gallin J.I. (Ed.), Principles and Practice of Clinical Research. 2002:267-272.
- Varni JW, et al. The PedsQL: Measurement Model for the Pediatric Quality of Life Inventory. Medical Care, 1999; 37(2):126-139
- Varni, J.W., et al. The PedsQL Version 4.0 Generic Core Scales in healthy and patient populations. Medical Care, 2001; 39(8): 800-812.
- Varni, J.W., et al., (2002). The PedsQL
- Landis JR, Koch GG (1977). The measurement of observer agreement for categorical data. Biometrics, 33: 159-74.
- Miller DC (1991). Handbook of research design and social measurement. Newbury Park, CA: Sage.
- George D, and Mallery P (2003). SPSS for Windows step by step: A simple guide and reference. 11.0 update (fourth edition). Boston: Allyn & Bacon.
- Moorthy, LN, Saad-Magalhaes, et al (2013). Validation of the Portuguese Simple Measure of Impact of Lupus Erythematosus in Youngsters (SMILEY) in Brazil. Lupus, 22: 190-197.
- Achenbach TM, McConaughy SH, Howell CT. Child/Adolescent Behavioral and Emotional Problems: Implications of Cross Informant Correlations for Situational Specificty. Psychol Bull 1987;101:213-32.
Additional Info
-
Language:
English -
Contains Audio:
No -
Content Type:
Articles -
Source:
ISN -
Year:
2015 -
Members Only:
No
